Fermentation converts a large fraction of wort sugars into carbon dioxide as well as ethanol. Only a small portion of that CO₂ is required for beer carbonation; the remainder is a valuable by-product that can be captured, purified, stored, and reused across the brewery or sold as food-grade gas. Capturing CO₂ reduces operating costs, improves sustainability, and supplies a consistent, on-site process gas for tank blanketing, packaging, dilution carbonation, and other applications.

Why recover CO₂? — Key advantages
Reduced purchase costs and improved margins
Capturing and reusing fermentation CO₂ reduces or eliminates the need to buy bulk industrial or food-grade CO₂, delivering direct cost savings and improving gross margins—especially for high-volume fermentations.
Reliable on-site gas supply
On-demand CO₂ supply reduces dependence on third-party deliveries, lowers logistical risk, and simplifies scheduling for packaging, tank purging, and other CO₂-dependent operations.
Multiple internal uses
Recovered CO₂ can be applied to:
- Carbonate dilution water in high-gravity brewing.
- Create counter-pressure for storage and transfer tanks.
- Strip dissolved oxygen from DE slurries and dilution water.
- Carbonate and top-up finished beer to specification.
- Flush and inert packaging lines and transfer piping.
Revenue potential
Where volumes and purity justify it, surplus CO₂ can be compressed and sold to external buyers (food, horticulture, non-food industrial), creating an additional revenue stream.
Sustainability and regulatory benefits
On-site recovery reduces the brewery’s carbon footprint from outsourced CO₂ production and logistics. It also supports sustainability reporting and may ease compliance with local emissions regulations.
Process and product quality control
Consistent, purified CO₂ reduces the risk of off-flavors or contamination in product handling and improves the quality of gas used for sensitive steps like low-DO dilution and packaging.
Alternative non-brewing uses
Recovered CO₂ can be repurposed for greenhouse enrichment, grain silo fumigation, or effluent neutralization—extending its value beyond the packaging line.
Where and when to collect CO₂
Primary source: fermentation vessel off-gas (highest volume and purity).
Secondary sources: storage/transfer tanks, packaging lines, filter housings, and any process points generating concentrated CO₂.
Timing: collection should be scheduled during the period when CO₂ concentration in off-gas is sufficiently high and oxygen levels are low—often several hours into active fermentation and during peak gas-off. Timing can be determined by SG drop, temperature rise, gas analysis, or a standard time band (e.g., 4–6 hours into active fermentation, adjusted by process data).
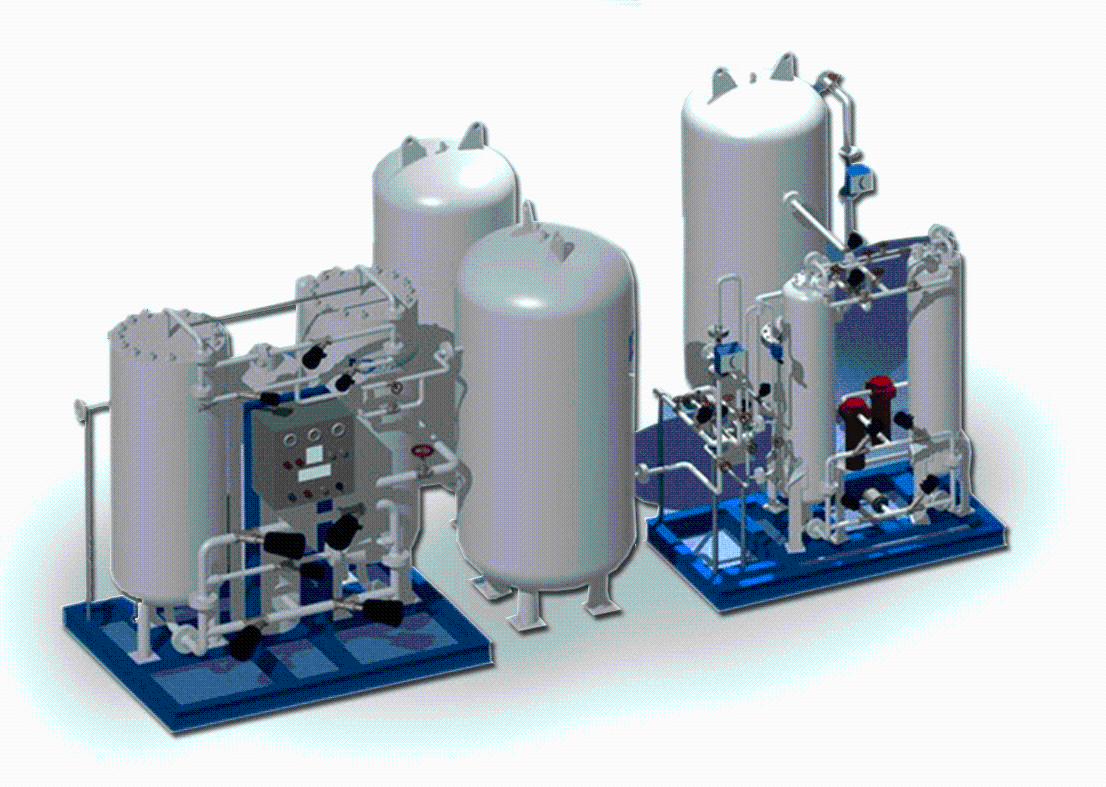
Typical purification flow
Removal of entrained liquids and solids
Methods: seal pots (water traps), scrubbers, cyclones. Purpose: protect downstream equipment and strip water-soluble volatiles (ethanol).
Oxygen reduction
Methods: staged cooling to condense CO₂ (CO₂ liquefies at −78°C; O₂ at −183°C), selective venting, or adsorption techniques. Purpose: avoid oxidation and meet food-grade specs.
Sulfur-compound removal
Methods: activated carbon beds or chemical scrubbers. Purpose: remove H₂S and other sulfur volatiles that affect flavor or gas quality.
Nitrogen / NOx removal
Methods: catalytic conversion (palladium beds) with controlled H₂ addition to convert NOx → N₂ + H₂O. Purpose: meet purity specs and prevent downstream catalyst poisoning.
Drying (water removal)
Methods: desiccant dryers (activated alumina, silica gel), molecular sieves, or cold traps. Purpose: prevent ice formation during liquefaction and ensure dry, stable stored CO₂.
Final steps: compression, liquefaction, storage in insulated cryogenic or pressurized tanks, continuous purity monitoring, and distribution piping with automated pressure/flow control.
Equipment and system recommendations
CO₂ capture headers and vent piping: collect from fermenters, tanks, and packaging points.
Seal pots and scrubbers: first-stage entrainment removal.
Gas coolers and condensers: staged refrigeration for O₂ separation and CO₂ liquefaction.
Activated carbon and catalytic converter modules: sulfur and NOx removal.
Desiccant dryers & molecular sieves: final drying before liquefaction.
Compressors & liquefaction units: for pressurization and storage.
Storage tanks (liquid/pressurized) with vapor return: stable supply and distribution.
Gas analyzers & continuous purity monitoring: O₂, H₂S, hydrocarbons, moisture sensors.
Integration & automation: PLC/SCADA control for scheduling, safety interlocks, and flow control.